A sickle cell disease treatment has been approved by the FDA and this could be life-changing news for the 100,000 Americans affected by the disorder. Most of those impacted by sickle cell disease are Black.
The name of the treatment is Casgevy and it’s the first medicine to be approved in the United States that uses CRISPR, a gene editing tool from Vertex Pharmaceuticals and CRISPR Therapeutics. In 2020, the creators of the tool won the Nobel Prize in chemistry. A patient’s blood cells are modified to remove the gene that causes the disease. The treatment has been approved for those ages 12 and older with recurrent vaso-occlusive crises.
In terms of how the treatment works, it’s classified as a one-time treatment but multiple extensive phases are involved. It requires numerous blood transfusions over the course of three to four months. Once that’s complete, stem cells are taken from the patient’s bone marrow and sent to a lab where they’re modified. Chemotherapy is then used to eliminate the patient’s bone marrow and ensure there are no flawed stem cells before they can receive the updated ones. Once the stem cells are reinfused, patient’s must stay in the hospital for a short period of time so the cells can grow. The clinical trial has been performed on 44 people in the U.S. and abroad; of the 31 patients “with sufficient follow-up time to be evaluable,” 29, or 93.5 percent according to Vertex, “achieved the primary outcome.”
“I am so proud to be part of the Vertex team as we receive this historic approval,” says Marjorie Dejoie-Brewer, the director of medical affairs for Vertex, in a statement. “We are entering a new era for the treatment of sickle cell disease after decades of mistreatment and being ignored. As a fellow warrior, my wish is that this will provide hope for the future, and a better chance for the Sickle Cell community to be seen and heard.”
“Sickle cell disease is a devastating, life-shortening disease. I witnessed this as a hematologist, knowing that I didn’t have many tools available to intervene. Which is why I am incredibly excited today, knowing that there are new options that may bring hope for some patients,” adds Bill Hobbs, hematology clinical development lead for Vertex.
In addition to Casgevy, the the FDA also approved a different gene therapy from Bluebird Bio called Lyfgenia that aims to eliminate pain crises and was also approved for treatment of SCD in those 12 years and older. It received a black-box warning, or a safety warning label, letting people know that in testing for this treatment, there were a few rare situations where the therapy could cause some blood cancers as two patients who received Lyfgenia via clinical trial died from a form of leukemia. It’s unclear though if the treatment was actually the cause or chemotherapy, which is known to be a risk factor in causing the cancer.
“People living with sickle cell disease need and deserve treatment options. To have two transformative therapies approved for this community, after nearly a century of underinvestment, is truly remarkable,” shares Bluebird Bio CEO Andrew Obenshain in a statement. “We have been working toward this moment for nearly a decade at bluebird, and know that these approvals would not have been possible without the determination, insight and partnership of the sickle cell community, who has fought tirelessly for investment and advances in in sickle cell care for so many years.”
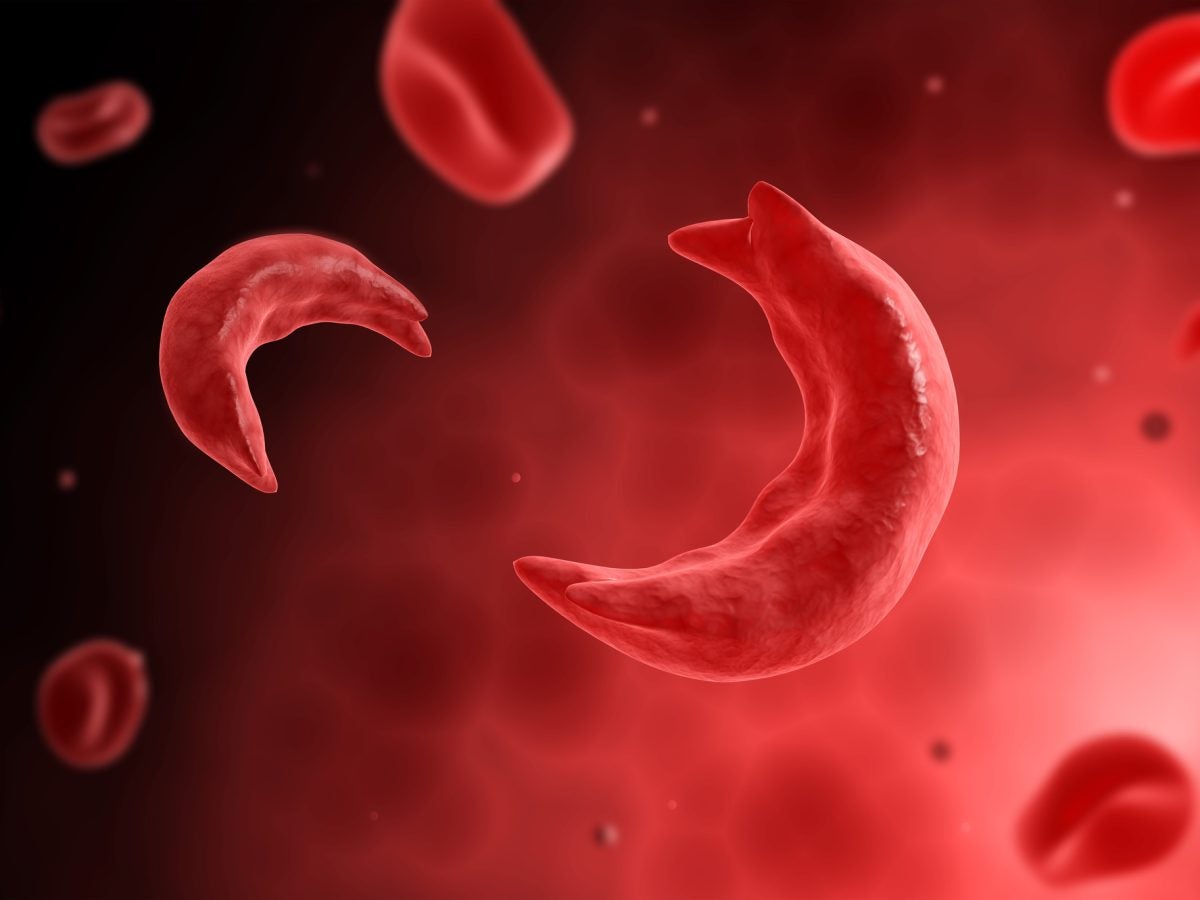
Sickle cell is a disease that affects the shape of red blood cells, which transport oxygen throughout the body. Red blood cells are typically round so they can easily move through the body, but SCD patients have crescent-shaped blood cells, which can disrupt their blood flow. Side effects include clots in blood vessels since oxygen can’t freely flow, leading to excruciating pain, trouble breathing or even strokes for SCD patients.
Up until now, the only cure for sickle cell disease has been a bone marrow transplant from a donor, which can be tedious for several reasons. For one, patients need to find a match and secondly, their body has to accept the transplant. Other than this, there has only been pain relief and treatments to help prevent the occurrence of complications. Both treatments alleviate the need for a donor, which could make a cure more accessible to some.
While this news may bring hopes to thousands of Americans, the treatments do have an expensive price tag, costing $2.2 million per patient for Casgevy and $3.1 million for Lyfgenia. That figure doesn’t include the cost of care during and after the treatment. Some may argue that for these reasons, these treatments are inaccessible to everyday people. But it is progress, and it’s the kind that gives people with sickle cell disease, as well as advocates, hope for more options in the future.
“The FDA’s approval of these gene therapies is an exciting and potentially curative addition to the treatments available to sickle cell warriors. This is a historic milestone, full of hope and promise for those living with the disease and their caregivers,” says Regina Hartfield, president and CEO of the Sickle Cell Disease Association for America, Inc. “But it is important to remember that gene therapy may not work for everyone with sickle cell disease. The Sickle Cell Disease Association of America, Inc. and our 50-plus member organizations nationwide are committed to ensuring research continues to move forward so that there is a therapeutic solution for every member of our community.”